We know you are busy…
…which is why we want to help reduce your workload
Your contribution as a clinical research investigator is invaluable in bringing new medicines to the patients who need them. But participating in clinical trials brings with it extra administrative tasks that add to the workload of already busy physicians and clinic personnel.
How can the Investigator Databank benefit investigators?
- Reduce individual requests for basic site information for each sponsor.
- Reduce redundant GCP training because sponsors will recognize GCP training conducted by other participating sponsors.
- Free up your time to focus on clinical activities.
- Expand your access to clinical research opportunities by making you & your site known to a broader base of trial sponsors.
Helps the busy sites like us to use a centralized data bank to timely update the site information. I wish other pharmaceutical companies will also support this effort.
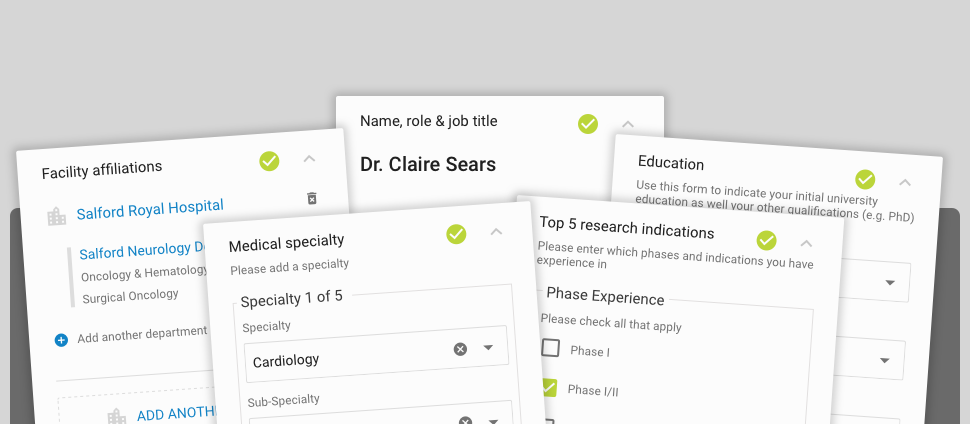
What kind of information will be included in the Investigator Databank?
Typical information that you routinely share with sponsors about trial site infrastructure, GCP training records, past trial participation, and recruitment history will be included in the Investigator Databank.
How will the information be used?
For consenting investigators, the information will be shared among all participating pharmaceutical companies to identify potential sites for upcoming trials. The data are also used for reference purposes, to set recruitment targets and timelines, and to reference GCP training records of other sponsors to support waiving of GCP training requirements if possible. Participating companies will not share your information with one another unless you “opt-in.”
What does it cost for me to participate in the Investigator Databank?
Nothing. There is no charge to investigators or their institutions to opt-in to the Investigator Databank and to create a profile. Industry member companies share the financial support for development and access to the Databank’.
Are you a site administrator?
Do you manage multiple physicians in the same clinic or across multiple clinics? Although each individual investigator needs to agree to opt-in separately, we can support you in coordinating this process, and in becoming the central point of contact for your investigators.
Contact us for more information