Investigator Databank Overview
The Investigator Databank is a global collaboration between pharmaceutical companies that aims to reduce administrative burden for investigators and to increase visibility of qualified investigators to research sponsors. Hosted by a 3rd party, DrugDev, the Investigator Databank is the one place where pharmaceutical companies can share investigator and site information and investigators can view, edit, and comment on their own information.
Current members of the Investigator Databank include:
AbbVie, Amgen, AstraZeneca, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Genmab, Gilead Sciences, Janssen, Jazz Pharmaceuticals, Lilly, Merck Sharp & Dohme LLC, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche, Sanofi, Takeda and UCB.
How does the Investigator Databank work?
Industry members share information from their clinical trial management systems including investigator/site contact details, GCP training records, past trial participation, and recruitment history. Upon investigator opt-in, this information is used by each participating company to identify sites for upcoming studies; to help set recruitment targets and timelines; and to share start-up information such as GCP training, CV details and and facility information. Typical information that you routinely share with sponsors about trial site infrastructure, GCP training records, past trial participation, and enrolment will be included in the Investigator Databank. No patient-level data are shared.
Who is in the Investigator Databank?
| Europe | 40% |
|---|---|
| North America | 36% |
| AsiaPac | 17% |
| Central/South America |
6% |
| Africa | 1% |
I am very excited and happy with this news. For a long time I have claimed and written, while filling feasibilities, about the loss of my time. I believe the steps taken as enumerated would help all the stake holders in the drug development.
Am I in the Records of a Member Company or DrugDev?
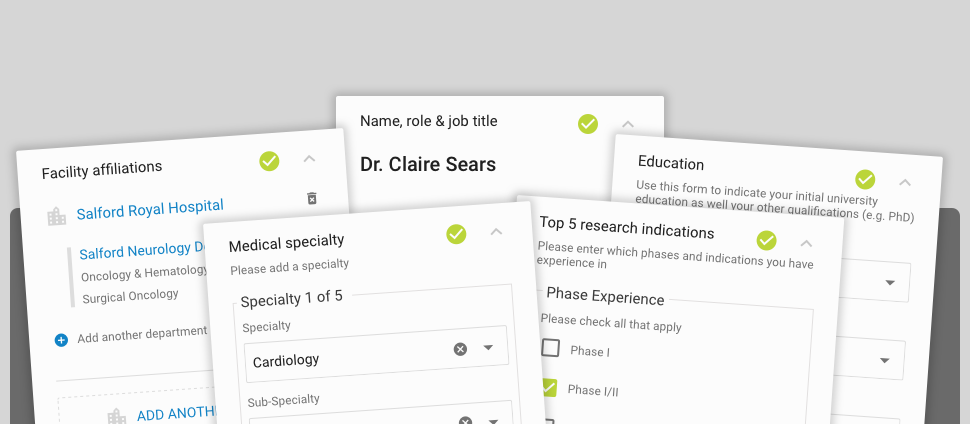
Become a part of the Investigator Databank to decrease your administrative burden on study start-up; increase your visibility to more industry sponsors for study opportunities; and view, edit, and comment on your information stored in participating company clinical trial management systems.
Create a Profile